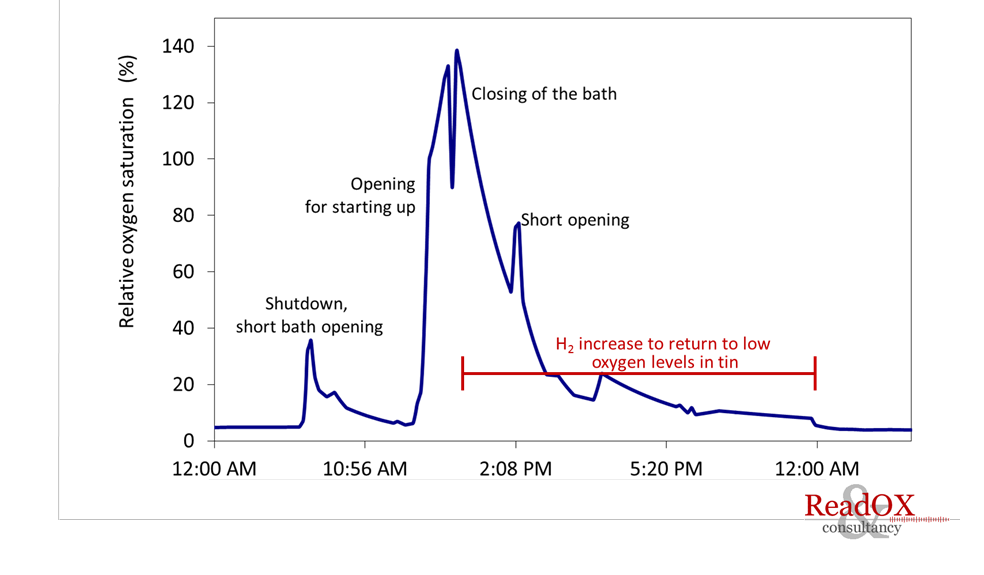
One of the major concerns in the operation of the tin bath is to prevent the oxidation of the tin melt. For this purpose the bath is provided with a positive pressure protective atmosphere consisting of a mixture of 1-10% hydrogen in nitrogen (forming gas). Oxygen may enter the furnace by air leaks in the bath perimeter, by diffusion from the glass sheet or as impurity in the forming gas. Oxygen levels in tin exceeding only a few ppm may already lead to various top and bottom surface defects, negatively affecting the production efficiency and/or the value of the final glass product.

The oxygen solubility in the tin bath is strongly dependent on the temperature. When the oxygen saturation level at a certain temperature is exceeded, tin dioxide (SnO2) will be formed on the surface of the tin melt (so-called dross), mainly occurring in the cold end section as oxygen solubility is low. Additionally, at the elevated temperatures in the hot end section, volatile tin monoxide (SnO) may easily evaporate from the bath forming Sn and SnO2 deposits on the colder overhead equipment and roof sections.

High oxygen levels in tin melt and consequently higher SnO2 and SnO levels on the tin surface and in the atmosphere, may lead may lead to bottom surface defects such as bloom and tin pickup, and top surface defects such as top tin, top speck and crater drip. All these oxygen related defects negeatively affects production efficiency and product value. Moreover, with the devellopment of high added value coating, a minimal distortion of the surface will lead to quality problems, making oxygen control even more important.

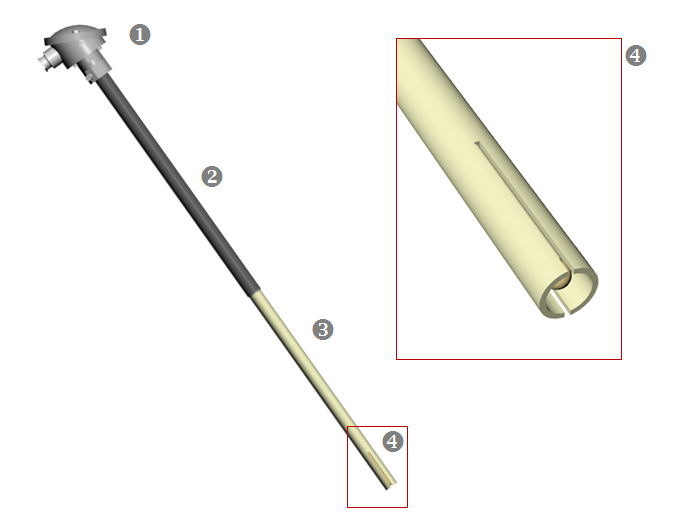
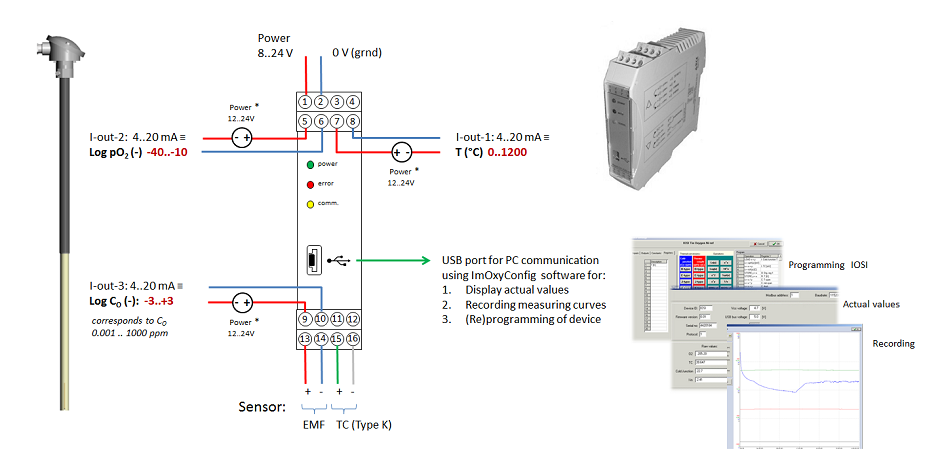
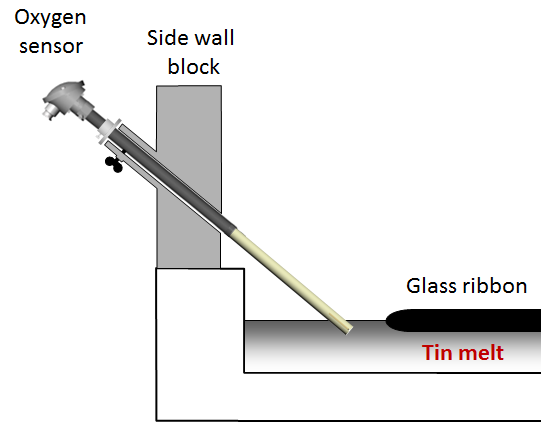 The geometry of the tin melt oxygen sensor is similar to that of a regular thermocouple. This allows simple replacement of the existing thermocouples along the entire tin bath length. The measuring tip of the tin melt oxygen sensor is in contact with the tin melt, similar to a tin thermocouple. The built-in K-type t/c of the oxygen sensor takes over the temperature measurement at the specific measuring location. Due to its internal solid reference, flushing of the zirconia cell with a reference gas is not needed. The large aluminium terminal head provides much space for convenient connection of the signal cables (t/c mV-signal and oxygen cell mV-signal) to the
The geometry of the tin melt oxygen sensor is similar to that of a regular thermocouple. This allows simple replacement of the existing thermocouples along the entire tin bath length. The measuring tip of the tin melt oxygen sensor is in contact with the tin melt, similar to a tin thermocouple. The built-in K-type t/c of the oxygen sensor takes over the temperature measurement at the specific measuring location. Due to its internal solid reference, flushing of the zirconia cell with a reference gas is not needed. The large aluminium terminal head provides much space for convenient connection of the signal cables (t/c mV-signal and oxygen cell mV-signal) to the